Sharing and reuse of individual participant data from clinical trials: principles and recommendations | BMJ Open

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine
How should individual participant data (IPD) from publicly funded clinical trials be shared? | BMC Medicine | Full Text

CONSORT Statement on Twitter: "Deb Zarin explains how poorly researchers understand what it means to share #IPD when asked what their sharing plan is #PRC8 https://t.co/TRSqa3Tx5S" / Twitter

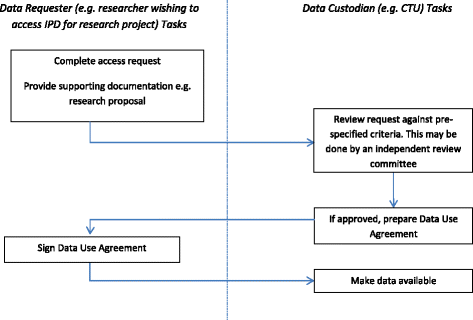
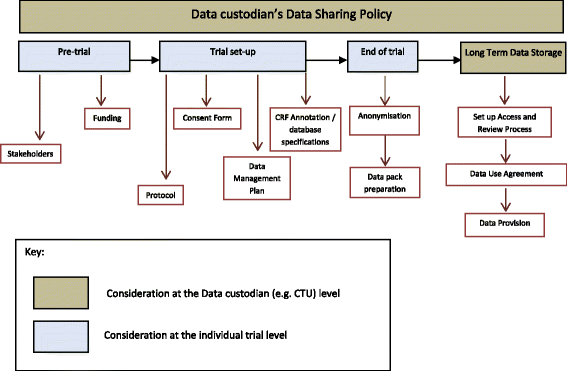
Sharing data from clinical trials: the rationale for a controlled access approach – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science

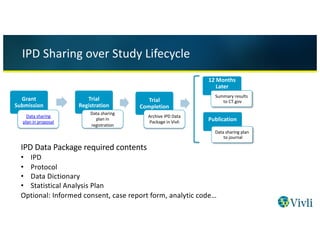
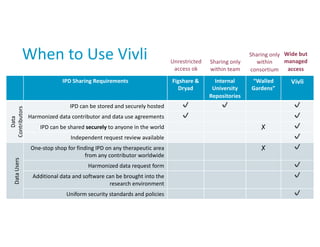
Overview and experience of the YODA Project with clinical trial data sharing after 5 years | Scientific Data

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine